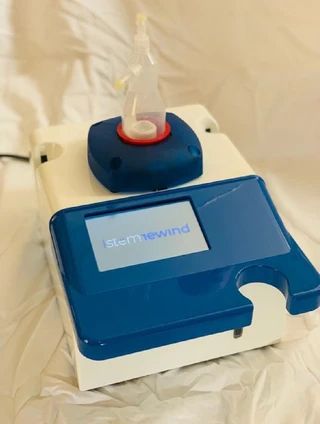
GenLife is a MedTech company commercializing IStemRewind, a patented, closed and sterile system that isolates adipose-derived stem cells in under 5 minutes, directly in the operating room. CE-marked for veterinary use and advancing EU MDR for human applications, IStemRewind standardizes regenerative workflows for osteoarthritis and complex wound care. Our model combines actuator sales with high-margin disposables and a scalable KOLdriven market entry.
Regenerative procedures are slow, costly and hard to standardize; labs and cultured cells trigger complex regulation and high OPEX.
• CE veterinary obtained; human CE target 2025; TRL 8
• Patents device and process EU and India; peer-reviewed in Biomedicines
• Funding to date 1.5M EUR self-funded plus 220k EUR early income
• 2024 revenue approx 70k EUR; 2025 projection approx 300k EUR
• Unit economics Actuator 25k EUR Disposables 1.5k EUR COGS approx 60 EUR
High burden of osteoarthritis and diabetic chronic wounds; demand for cost-effective intraoperative solutions.
Regulatory path via CDSCO; local pilots enable rapid validation and KOL engagement.
Partner models: JV build + distribute; OEM or ODM exclusive distribution; import to localize year one then local build.
Patented device and process; enzyme-free closed sterile workflow in under 5 min. Validated with ISO 13485 suppliers; CE veterinary obtained; human CE in progress; TRL 8.
Designed for reproducibility and OR integration; low perprocedure cost and very short staff time.
Successful proof of concept with an international pharma: technology, device, QA and ISO 13485 manufacturing due diligence.
Peer-reviewed publication in Biomedicines; positive KOL feedback in orthopedics and wound care